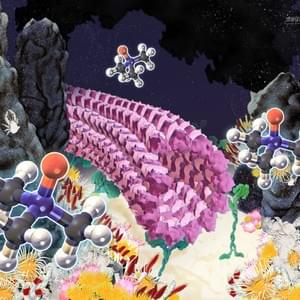
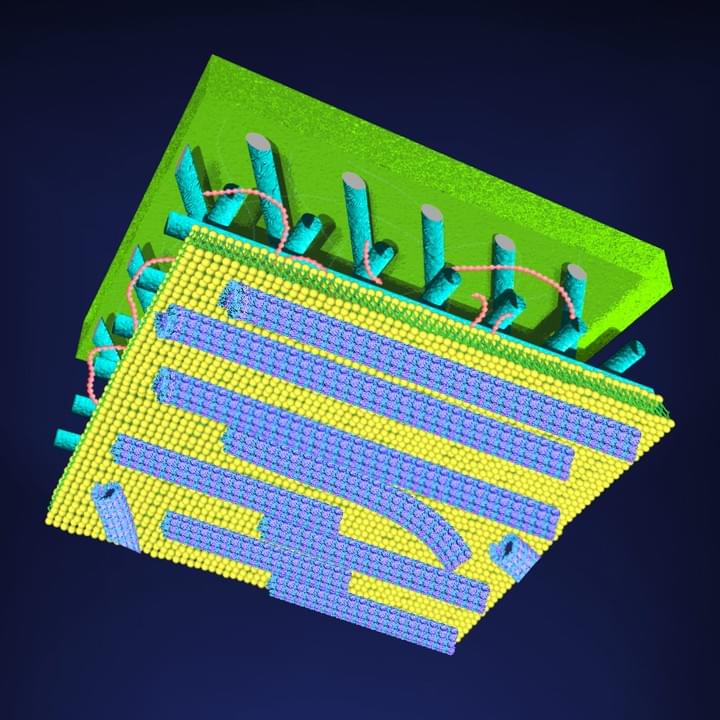
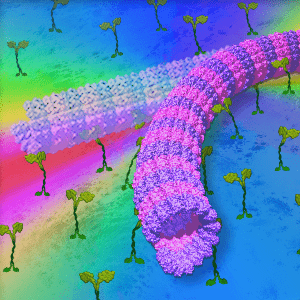
Cytoskeletal Architecture
and
Bio-Self-Assembly lab
Design inspired by self-organization in biosystem
Self-organization is a process in which local interaction between components of a system spontaneously produce ordered structures and fascinating patterns which exhibit emergent functions in the absence of a professional designer. This is a common assembly process in a biosystem and can be co-opted by scientists and designers based on different construction principles and logics of design. Since self-organization grants several advantages to the system including robustness, flexibility and adaptability to the surrounding environment, innovations based on this self-organization may provide a unique avenue for designers and inventors.

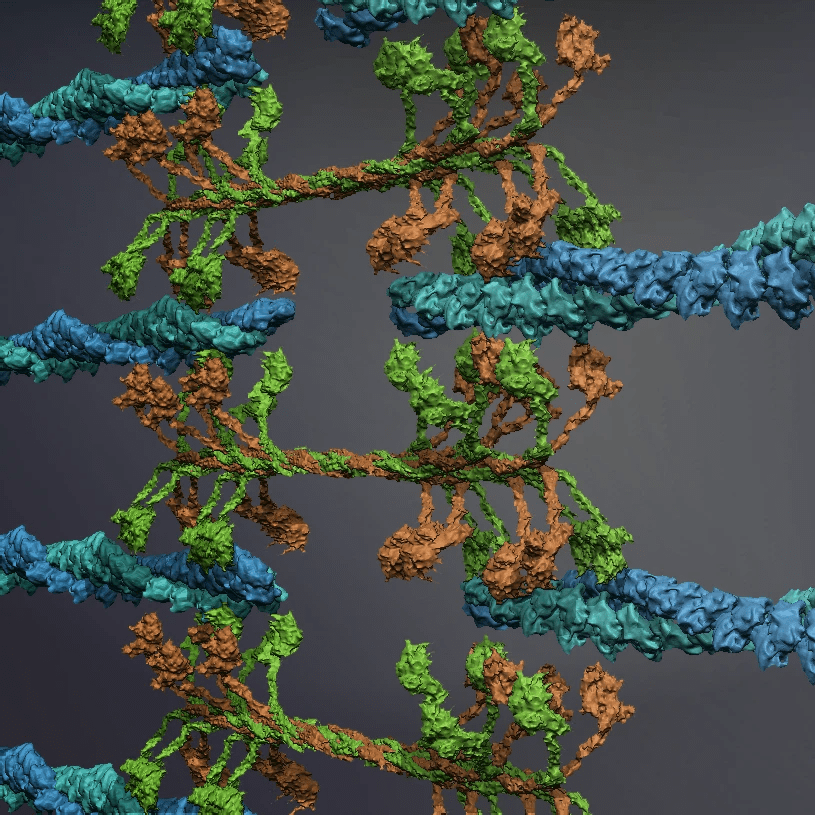
Biomaterials: Cytoskeletons & Motor proteins
Biophysics, Micro/Nanotechnology
The study of self-organization in a biosystem, a system comprised of skeletons in cells so-called as cytoskeleton (microtubules and actin) and its related motor proteins, provides one with unique tools and design principles not seen in other systems. The biosystem is one of the best candidates for innovation since they autonomously form various structures, and determine the morphology of the cell wherein they lie. This allows a designer with the ability to innovate with these self-assembling structures, potentially bringing this microscopic design into the macroscopic world. We are studying it using the reconstituted system of cytoskeleton combining with micro/nanotechnologies.
Artificial materials
Interior, Architecture design
To explore potential applications of self-organization, our other challenge is to develop artificial systems organized through self-organization at human scales inspired by cytoskeletons.
Basic Equipments & technologies

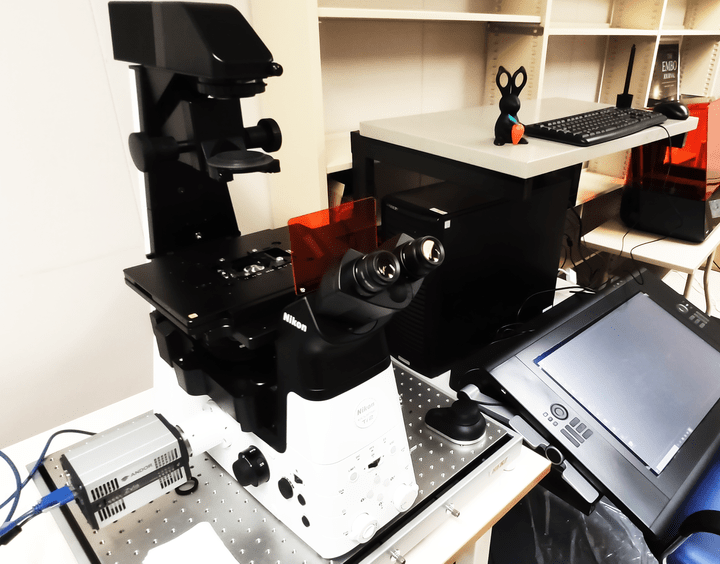
Nikon Ti2-E,
Epi-fluorescence microscopeFluorescence microscopy imaging

Optline TIRF ReLIEF
A total internal reflection fluorescence microscope to observe thin region of a specimen, usually less than 200 nanometers

SPM/AFM SPA300HV
Atomic force microscope to visualize surface molecular structure of materials at nm resolution

Formlabs Form3,
3D printer
3D print at high resolution
(0.025 mm) using Low Force Stereolithography (LFS)Selected Papers

Surface Passivation of Norland Optical Adhesive Improves the Guiding Efficiency of Gliding Microtubules in Microfluidic Devices
Inoue, D.

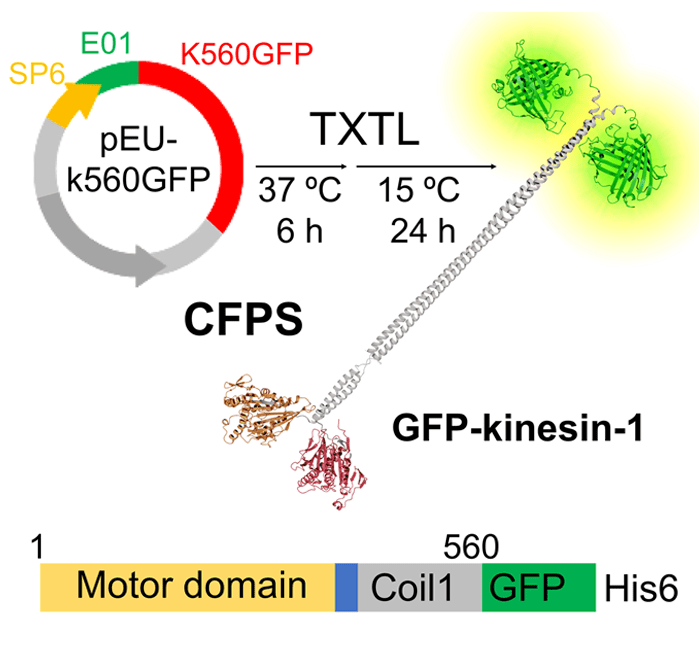
In Vitro Synthesis and Design of Kinesin Biomolecular Motors by Cell-Free Protein Synthesis
Inoue, D.; Ohashi, K.; Takasuka, E. T.; Kakugo, A.
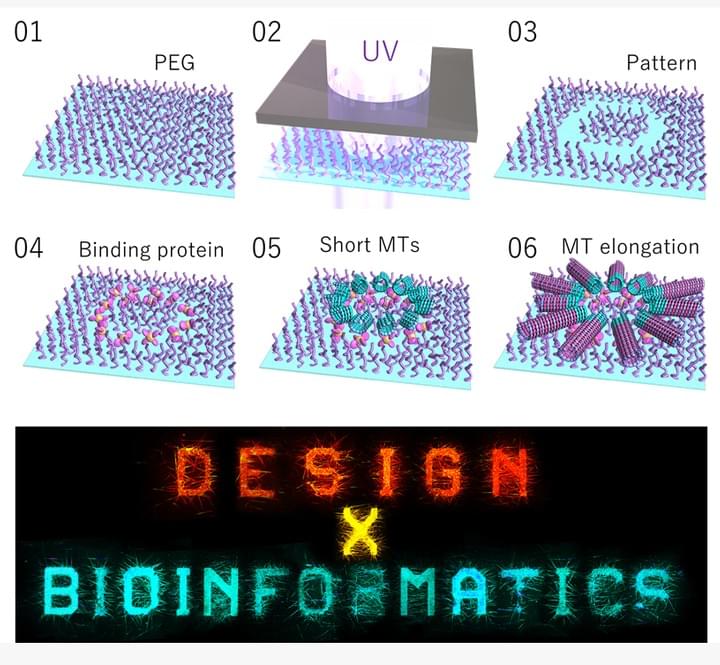
 Design X Bioinformatics: a community-driven initiative to connect bioinformatics and design
Design X Bioinformatics: a community-driven initiative to connect bioinformatics and designSommer, B.*; Inoue, D.; Baaden, M.
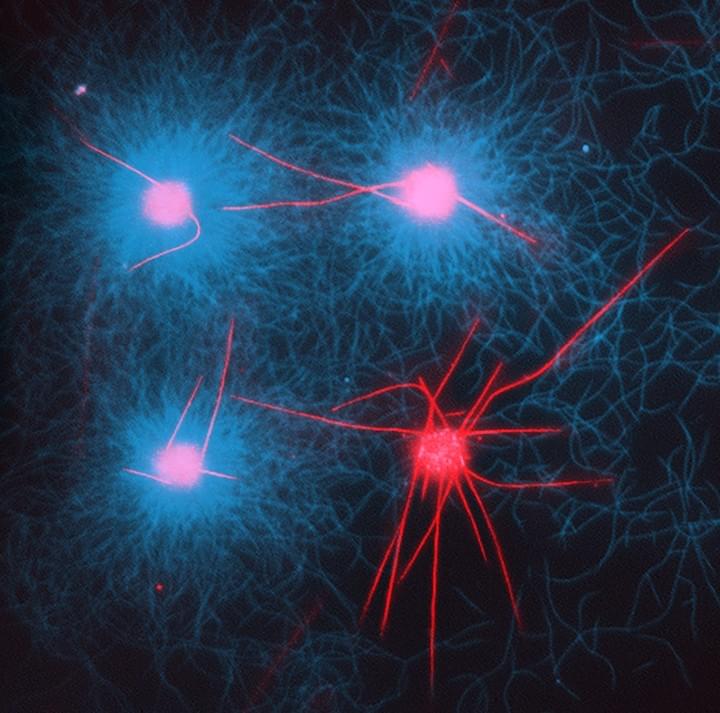
 Monopolar flocking of microtubulesin collective motionAfroze, F.†; Inoue, D.†; Farhana, T. I.; Hiraiwa, T.; Akiyama, R.; Kabir A. M. R.; Sada, K.; Kakugo, A.*(†Contribution equal)
Monopolar flocking of microtubulesin collective motionAfroze, F.†; Inoue, D.†; Farhana, T. I.; Hiraiwa, T.; Akiyama, R.; Kabir A. M. R.; Sada, K.; Kakugo, A.*(†Contribution equal)
Self-repair protects microtubules from destruction by molecular motors
Triclin S.†, Inoue D.†, Gaillard J., Htet Z. M., DeSantis M. E., Portran D., Derivery E., Aumeier C., Schaedel L., John K., Leterrier C., Reck-Peterson S. L., Blanchoin L.* & Théry M.* (†Contribution equal)

Mechanical stimulation‐induced orientation of gliding microtubules in confined microwells
Inoue, D.; Kabir, A. M. R.; Tokuraku, K.; Sada, K.; Kakugo, A.

Adaptation of patterns of motile filaments under dynamic boundary conditions
Inoue, D.; Gutmann, G.; Nitta, T.; Kabir, A. M. R.; Konagaya, A.; Tokuraku, K.; Sada, K.; Hess, H.; Kakugo, A.

Actin filaments regulate microtubule growth at the centrosome
Inoue, D. †; Obino, D.†; Farina, F.; Gaillard, J.; Guerin, C.; Blanchoin, L.*; Lennon-Duménil, A. M.*; Théry, M.*

Are microtubules tension sensors?
Hamant*, O.; Inoue, D.; Bouchez, D.; Dumais, J.; Mjolsness, E.

Construction of artificial cilia from microtubules and kinesins through a designed bottom-up approach
Sasaki, R.; Kabir, A. M. R.; Inoue, D.; Anan, S.; Kimura, A. P., Konagaya, A.; Sada, K. and Kakugo, A.*

DNA-assisted swarm control in a biomolecular motor system
Keya, J.; Suzuki, R.; Kabir, A. M. R.; Inoue, D.; Asanuma, H.; Sada, K.; Hess, H.*; Kuzuya, A.*; Kakugo, A.*

Depletion force induced collective motion of microtubules driven by kinesin
Inoue, D.; Mahemuti, B.; Kabir, A. M. R.; Farhana, T. I.; Tokuraku, K.; Sada, K.; Konagaya, A.; Kakugo, A.*

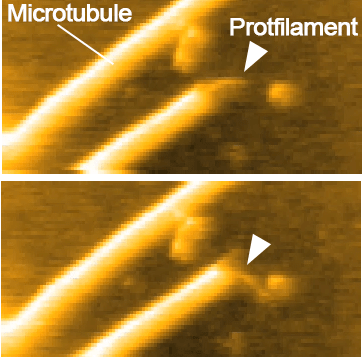
High-resolution imaging of a single gliding protofilament of tubulins by HS-AFM
Keya, J. J.†; Inoue, D.†; Suzuki, Y.; Kozai, T.; Ishikuro, D.; Kodera, N.; Uchihashi, T.; Kabir, A. M. R.; Endo, M.; Sada, K.; Kakugo, A.* (†Contribution equal)

Sensing surface mechanical deformation using active probes driven by motor proteins
Inoue, D.; Nitta, T.; Kabir, A. M. R.; Sada, K.; Gong, J.P.; Konagaya, A.; Kakugo, A.*
Free Materials for BioArt
Anyone can use those materials for free. But please don't redistribute these items without modification.
Member

Contact
Kyushu University, Ohashi campus
Room 605, Building 3, Shiobaru 4-9-1, Minami-Ku, Fukuoka, 815-8540, Japan(+81) 92-553-4431
© 2019